Ensuring Superior Quality to meet Industry Standards and Build Trust
Quality in Manufacturing
Wembrace Biopharma is committed to maintaining the highest standards of product quality at its manufacturing plant through a well-rounded Quality Management System (QMS). Our quality cytotoxic drugs are being supported by award winning WHO-GMP certified manufacturing plants complying to OEL-5, UKMHRA & USFDA standards. Our ‘state of the art’ plant is powered by a fully automatic Italian machine from IMA, Italy. The world pioneer in cutting-edge automation technology that guarantees higher quality and compliance by minimizing human error.
Here’s a closer look at the commitment to crucial parameters listed below:
QMS:
The implementation of a well-structured QMS serves as the cornerstone for preserving and continually elevating product quality. By ensuring unwavering adherence to quality policies, procedures, and standards throughout the manufacturing process, this commitment assures consistent product quality.

Product Performance:
The continuous monitoring of product performance, facilitated by rigorous testing and quality control measures, demonstrates a proactive approach to identifying any deviations from expected quality standards. This enables the prompt implementation of corrective actions to maintain product quality.
Delivery:
Ensuring timely product delivery showcases an understanding of customer expectations and effective management of production schedules and logistics to meet these expectations.
Quality Assurance
We also have an in-house Quality Assurance section that assures our manufacturing facilities comply with WHO GMP requirements. This department is responsible for and has the jurisdiction to create standards and rules for a variety of operations, including employee training, sanitization, and general area cleanliness. The team members go over the production records to ensure that all manufacturing processes are carried out according to the specifications. The Quality Assurance department is assigned with the following responsibilities:
- All validation procedures are being evaluated and approved.
- Specifying sampling methods
- Final SOP evaluation and approval
- Reviewing and approving all BMR/BPRs prior to the release of commodities for distribution
- Approving the batch release
- Ensuring GMP compliance throughout batch production
- Vendor auditing and approval
- Market complaints and batch recalls are evaluated and analysed.
- Ensure consistently finished product stability
- Analytical evaluations are carried out.
- Maintaining document control
- Approval of all completed items produced, processed, shipped, or stored by the firm
- Ensuring that all manufacturing facilities can fulfil GMP criteria and that all activities are carried out by appropriately trained and competent personnel
- Ensuring that all production procedures are well defined and periodically reviewed to ensure the quality of completed items
At Wembrace Biopharma, we take pride in our commitment to delivering pharmaceutical and biotechnological products of the highest quality. To support this commitment, we have established an in-house Quality Control Laboratory that is not only well-equipped but also self-sufficient, right within our manufacturing plant. This strategic integration offers a multitude of benefits that reverberate across our operations and, ultimately, to our valued customers.
- Timely Quality Assurance: Our in-house laboratory allows us to maintain rigorous quality standards at every stage of production. With real-time testing and inspections, we ensure that our products consistently meet and exceed industry benchmarks.
- Rapid Decision-Making: Immediate access to quality control data empowers us to make informed decisions swiftly. If any deviations or issues arise, we can take prompt corrective actions, mitigating the risk of substandard products and enhancing overall efficiency.
- Cost Efficiency: Over time, our self-sufficient laboratory proves to be cost-effective. We exercise precise control over testing expenses, optimizing resources according to our specific requirements while delivering exceptional value to our customers.
- Customized Testing: Our laboratory is tailored to meet the unique and exacting testing demands of biopharmaceutical products. This customization ensures that we focus on the critical tests that matter most to our processes.
- Seamless Data Integration: Data generated within our laboratory seamlessly integrates with our manufacturing systems. This integration supports advanced process control, comprehensive data analysis, and the continuous improvement of our production processes.
- Regulatory Compliance: We understand the paramount importance of regulatory compliance in the biopharmaceutical industry. Our in-house laboratory aligns seamlessly with stringent regulatory requirements, reducing compliance risks and safeguarding our reputation.
- Confidentiality and IP Protection: Our in-house setup enhances confidentiality and protects intellectual property. We maintain full control over sensitive product information and proprietary processes, safeguarding our competitive edge.
- Cultivating a Quality Culture: Our laboratory fosters a culture of quality throughout our organization. Our dedicated team takes ownership of quality control processes, consistently striving for excellence and innovation.
- Streamlined Communication: The proximity of our laboratory to the manufacturing floor facilitates efficient communication between quality control personnel and production teams. This ensures that any issues are addressed promptly and effectively.
- Integration for Efficiency: Integration of quality control directly within our manufacturing plant streamlines operations. This close collaboration allows for swift responses to quality issues, minimizing disruptions to our production processes.
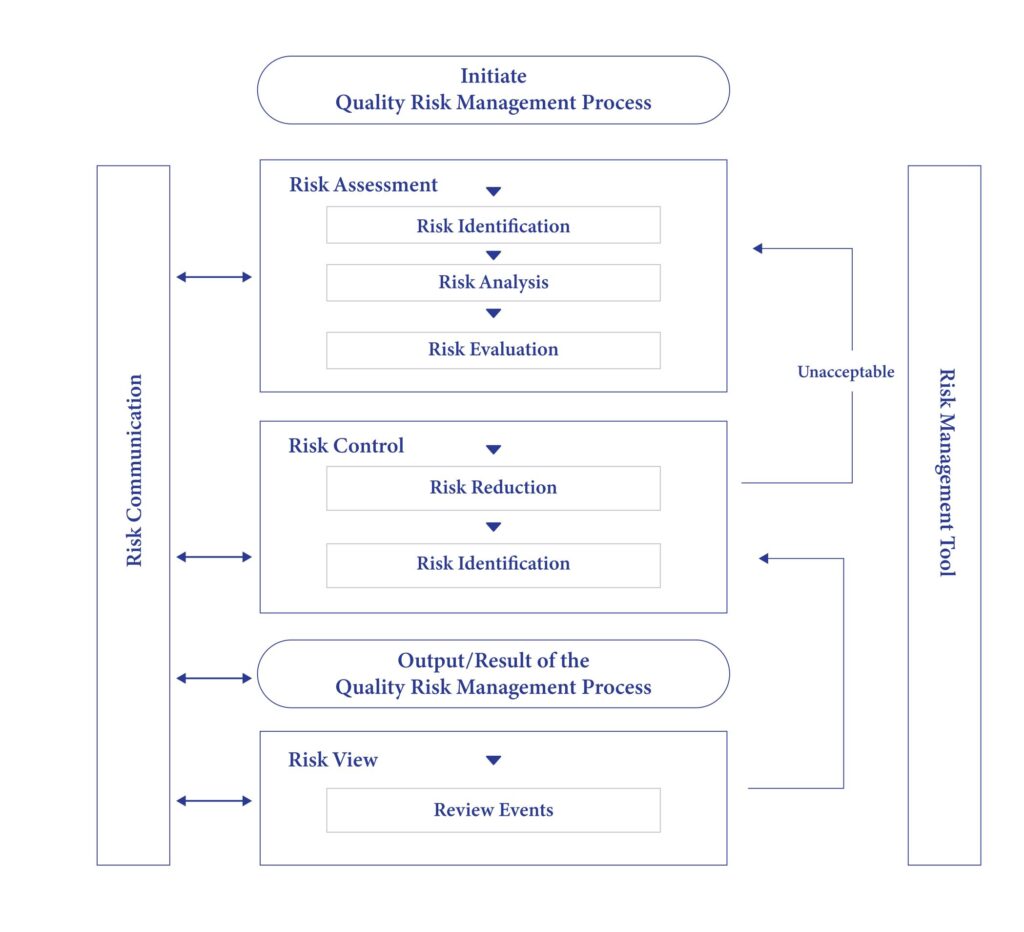
In addition, risks to product quality have been assessed in various ways and quality management practices as per ICH Q9 are followed.